LINX Magnetic Sphincter Augmentation for Reflux
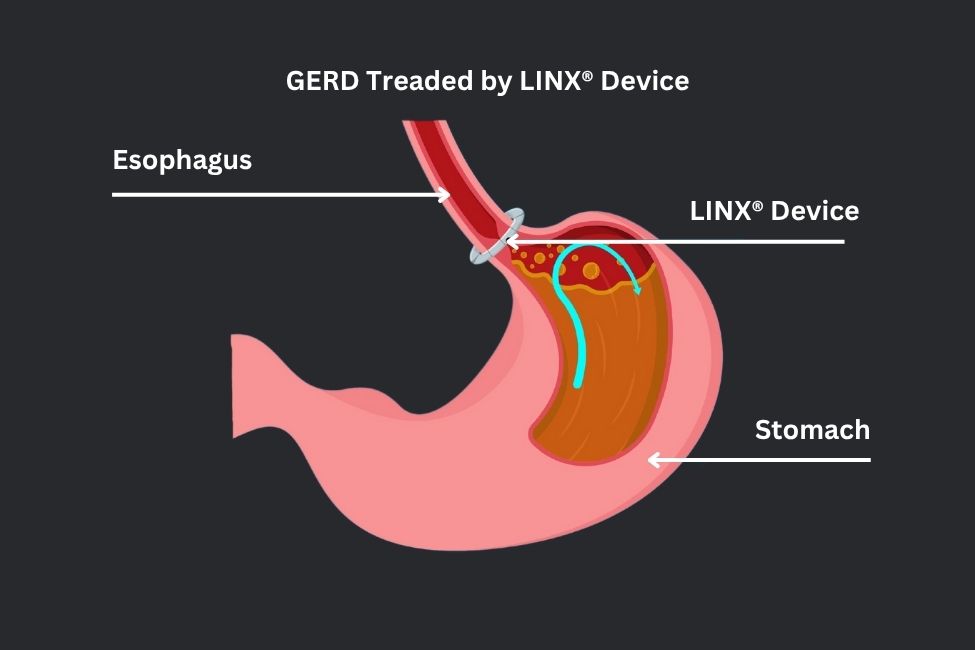
Our surgeons are the first in the area to offer the LINX® reflux management system for the treatment of GERD. Magnetic sphincter augmentation is a highly effective, minimally invasive option for the surgical treatment of GERD – and it is our procedure of choice for patients who are candidates. A magnetic sphincter augmentation device (LINX® device) is a small ring of magnetic beads that are placed around the lower esophagus and serves to “re-create” the lower esophageal sphincter. It is designed to open and close at a specific pressure, thus, preventing acid reflux from passing up into the esophagus, while allowing food to flow into the stomach. It also allows for patients to belch or vomit, if necessary – and it turns out this is quite important.
The surgery is performed laparoscopically, under general anesthesia, and is typically completed in an hour or so. Patients can generally go home the same day. Most patients report some degree of difficulty swallowing (dysphagia), after the device is implanted, which is a normal part of the healing process. This feeling should resolve over time but may last up to 12 weeks. Less than 3% of patients will ever need to have the device removed, usually in case of persistent dysphagia. If it comes to this, the device can easily be removed laparoscopically – generally with ongoing improvement in reflux control, even after the device is removed.
Patients usually start to notice an improvement in their GERD symptoms immediately, most can completely stop their acid suppression medication, and patients are generally extremely satisfied.
What is LINX®?
The LINX® Reflux Management System (LINX device) consists of a series of titanium beads, each with a magnetic core, connected with titanium wires to form a ring shape. The LINX® device is surgically implanted around the lower end of the esophagus.
Even though the LINX® device helps to prevent stomach contents from flowing back into the esophagus, it does not prevent the movement of food or liquids down the esophagus into the stomach. When the patient swallows, the pressure in the esophagus increases and the magnetic beads move apart on the titanium wires. As the beads move apart, the magnetic force decreases. This separation of the beads allows food or liquids to pass normally into the stomach. After the food or liquids have passed into the stomach, the magnetic beads return to the closed position.
When is LINX® used?
The LINX® device is an option for any patient who is a candidate for anti-reflux surgery. There are certain situations where you might not be a candidate for the LINX®, so this is something that will be determined when you see the surgeon for an evaluation.
What will LINX® accomplish?
The LINX® device will help prevent abnormal amounts of acid from the stomach from moving back into the esophagus. A growing body of literature supports the safety, efficacy and long-term durability of this treatment. In a recently published 5-year follow-up of the patients who had a LINX® device implanted, as a part of the FDA trial, there were no device erosions, migrations or malfunctions noted. Control of GERD symptoms was excellent, with regurgitation reported in only 1% of patients at 5 years. All patients reported the preserved ability to belch and vomit, if needed. Bothersome dysphagia (difficulty swallowing) was present in 5% before surgery and 6% at 5 years (“Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux“).
Patients who have the LINX® device should not be exposed to, or undergo, an MRI (Magnetic Resonance Imaging) in a magnet more powerful than 1.5 Tesla for the current version of the device. Exposure to the more powerful MRI could cause injury to the patient and the device may be damaged.
For more information on the LINX® device and whether this treatment might be right for you, contact our office.